We Speak Your Languages: Clinical Trial and Medical Imaging
At tWAN Biotech, we provide an extensive array of imaging services based on the cloud-native platform that caters to streamlining the trial workflows of surrogate imaging endpoints and quantitative imaging biomarkers for novel drugs, medical devices, and imaging AI, SaMD.
Imaging in Clinical Trials
We tailor our services to meet the technical and/or operational requirements on chosen imaging endpoints and the strategic needs of your clinical trial across imaging trial design, development of imaging charter and documents, imaging data management to image re-read and rescue study. Book a free consultation and contact us to learn how our imaging endpoint services can benefit your imaging trials.
EFFICACY
Efficacy Surrogate Endpoints
PFS of iRECIST, RANO 2.0
MRI-PDFF of Fatty Liver
SAFETY
Safety Endpoints
ARIA-E, ARIA-H associated with Anti-Amyloid Drug
Cardiotoxicity of CAR-T & Novel Oncological Therapeutics
PATIENT SELECTION
Quantitative Inclusion Criteria
CT Perfusion of Acute Ischemic Stroke
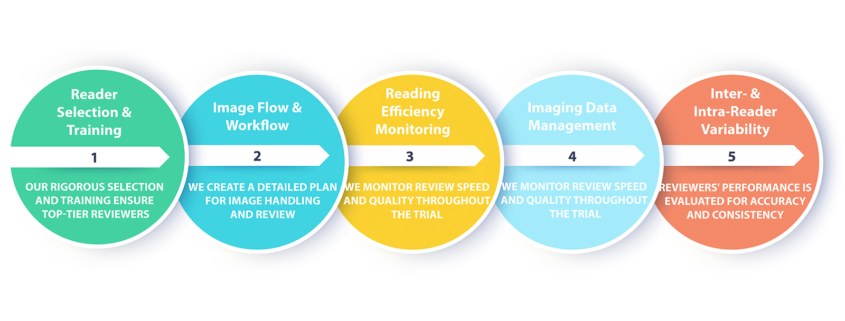
Central Reading (BICR) Management
As the leading Central Reading and BICR service provider in Taiwan, tWAN Biotech works closely with outstanding imaging professionals in different subspecialties. Multiple central readers with adjudication mechanism are seamlessly implemented into our central reading workflow.
Experience tWAN Biotech’s BICR solutions built onto our cloud platform that provides traceable quality measures and transparency to all parties to ensure consistent, precise and compliant reviews.
Cloud-based Imaging Solution
Our management platform harnesses the power of cloud-native technologies to streamline every aspect of imaging processes and reliably manage imaging related source data in clinical trials. From secure image transmission, structured review processes to customizable eCRF design, built-in audit trails, and seamless data integration, the platform optimizes the entire workflow.
Adhering to the latest data privacy regulations and security standards, the platform ensures the safeguarding of sensitive data while providing a real-time, audit-ready and reliable environment for imaging-based clinical trials.
Service Portfolio
What we offer:
- Central Reading & BICR Management: Central and Site Reader Training and Monitoring, Re-Read Management
- Cloud-based Imaging Management Platform
- Conduct of Imaging Operation in Clinical Trials: Imaging Site Monitoring and Training, Document Writing, Imaging Source Data Verification and Management
- Consultation on Imaging Trial Design and Imaging Endpoint Strategies for MOA, Exploratory & Confirmatory Trials
- Quantitative Imaging Services: Selection of Imaging Endpoint/Biomarker based on Evidence Level and Operability, Qualification/Validation of Quantitative Imaging Analytic Software
Therapeutic Areas
- Oncology including Immuno-Oncology
- Cardiovascular
- CNS including Neurodegenerative Diseases, Stroke
- Gastroenterology and MAFLD/MASF (NAFLD/NASH)
- Rheumatology
Imaging Modality
- CT
- MRI
- PET
- Ultrasound/Echocardiography
- Hybrid Scanners: PET/MR, PET/CT